Chemistry and biology are not separate worlds
BMSIS Scientist Omer Markovitch builds models to predict chemical speciation. (Following is an interview with Dr. Markovitch, translated from the NEMO Kennislink website)
Before there was life on earth, molecules already displayed behaviour that resembles life, such as reproduction and evolution. Omer Markovitch tries to understand and predict that behaviour using computer models.
How did life originate on earth? “That is far too big a question to investigate all at once,” says Omer Markovitch, a post-doctoral researcher at the University of Groningen and a Fellow at the Origins Center. “The transition from non-life to life is not black and white, but a multi-step process.”

You can approach the origins of life in many different ways. Where did you start with your research?
‘First, I will explain where I did not begin, and why. A commonly used approach is to begin with life as we know it today – for example, a simple bacterium – and simplify it as much as possible until they discover the minimum requirements. That gives you a very simple “minimal” cell, but it’s still much too large and too complicated to have arisen spontaneously.’
‘You could also start with the single molecules that we know existed on the young earth, even before there was life. They’re still important building blocks for life. Then you try to figure out how the first primitive living cell might have been formed from those building blocks. But, with that approach, you run the risk of tunnel-vision because you assume that the molecules that are important now were also the foundation for the very first life. There is no evidence for that. It’s possible that those molecules only became important bit by bit, and the starting point was somewhere else. If you are fixated on a certain set of molecules, you immediately rule out other possible explanations.’
So what approach are you left with?
‘I’m not trying to figure out the entire process from non-life to life. Instead, I’m concentrating on two steps in that process: reproduction and evolution. Everyone agrees that they are both essential characteristics of life. I want to understand how those processes work on a molecular level. How did those abilities arise? We know that even before life existed, all kinds of molecules could be formed. However, I don’t focus on specific molecules; I look at a more abstract level. What is necessary for various molecules to form larger structures that can multiply and adapt? For me, it’s all about chemical evolution: what does evolution mean at the molecular level?’
Does that resemble evolution as we know it from biology?
‘Yes, I think so. I also try to apply the principles from evolutionary biology to chemical systems. Chemistry and biology are not separate worlds; we are all part of the same reality, so I think the same rules should apply. The problem is that we don’t recognise those rules when we look at molecules.’
Can you discuss the behaviour of molecules in general terms? That behaviour is largely due to their specific characteristics. Even small differences between molecules can lead to completely different chemical behaviour.
‘The principles I’m looking for are related to the underlying relationships and interactions between molecules that form a network together. The way they react to each other, and especially the extent of those interactions, influences the behaviour of the entire system. Compare it to a living cell, in which an incredible number of reactions occur simultaneously, but some go very quickly at a certain moment, causing them to trigger or slow down other reactions. It’s a synchronised whole that can function accordingly. I’m looking for the emergent behaviour [behaviour that cannot be predicted from the individual components, ed.] of a network and I’m trying to discover which properties a system needs to show specific behaviour.’
What does your research look like in practice?
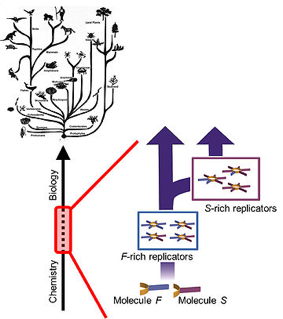
‘I’m mainly working on building and refining computer models that mimic chemical systems. I’m fortunate to collaborate with Sijbren Otto’s research group here in Groningen, so I can use real data from their experiments in my work. Those researchers have created a system of chemical replicators that spontaneously form larger rings. The rings then accumulate into something like small fibres that, in turn, exhibit interesting behaviour. That system is the starting point for my models. I then combine it with insights from evolutionary biology to discover the circumstances that are necessary to initiate chemical evolution.’
Can you give an example of a principle from biology that you use?
‘Speciation is a good example. Evolutionary biology teaches us that the availability and variety of food is an important driving force behind specialisation and speciation. If there is only one food source, the species that can consume that food the fastest and best will become dominant. There is no more room for other species. But, if there is a great variety of food, various species will likely develop because there is more space for specialisation and less competition for that single food source. Think of Darwin’s famous finches with the differently shaped beaks.’

How do you translate that to the molecular level?
‘You offer various building blocks that can all form rings and fibres – also in combination with each other – but that have different reaction speeds. The replicator that responds and consumes the fastest will initially become dominant, but what happens if it runs out of the preferred building block? Will it adapt or will another replicator take over, one that may grow more slowly but is less picky and can work with various building blocks? By continuously adjusting characteristics and conditions, I hope to discover the most decisive factors that allow speciation to occur.’
What do you base your choices on? How do you ensure that your models still relate to chemical reality?
‘It’s not completely random; my decisions are based on the data from chemical experiments performed in Otto’s research group. That’s my starting point. Then I choose replicators that differ slightly from the existing ones but are not so different that they won’t react to each other. You can make informed choices about that, but, since it remains a choice, you never know. There is continual exchange between the experiments in the lab and the results from my models, which I can use to improve my estimates and choices again and again.’
What do you ultimately hope to learn from your models?
‘My ultimate goal is to discover the universal rules of evolution.’
That’s quite ambitious.
‘Yes, and it’s not going to happen overnight. My project will run for another two years and I hope to learn a lot more. For instance, how can we choose the right conditions in a system with ten replicators that will ultimately lead to the creation of five species and further develop them? If I could predict the experimental conditions you need to achieve such a result, that would be a really huge step.’
Translation of ‘Chemie en biologie zijn geen gescheiden werelden’, an interview by Esther Thole from the NEMO Kennislink website (https://www.nemokennislink.nl/publicaties/chemie-en-biologie-zijn-geen-gescheiden-werelden/). Translated by Erin Goedhart-Stallings (www.stallings.nl). This translation is licenced under CC.BY-NC-ND 4.0.
